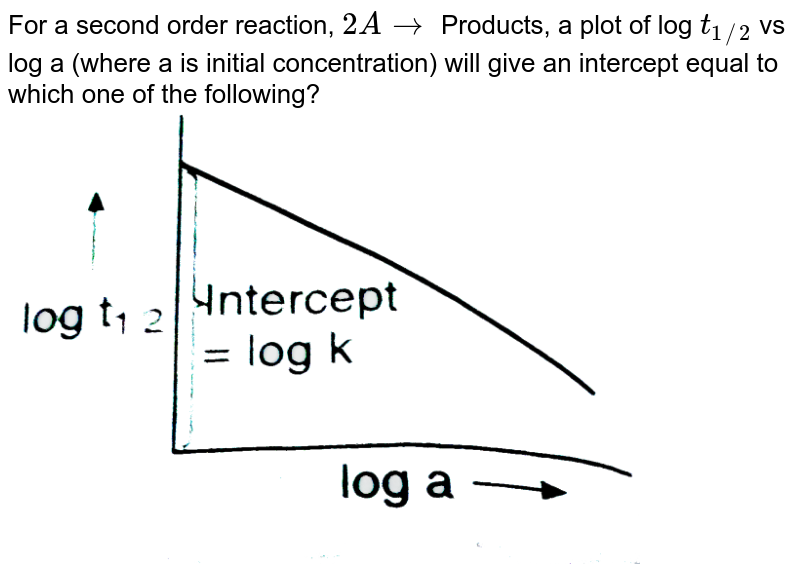
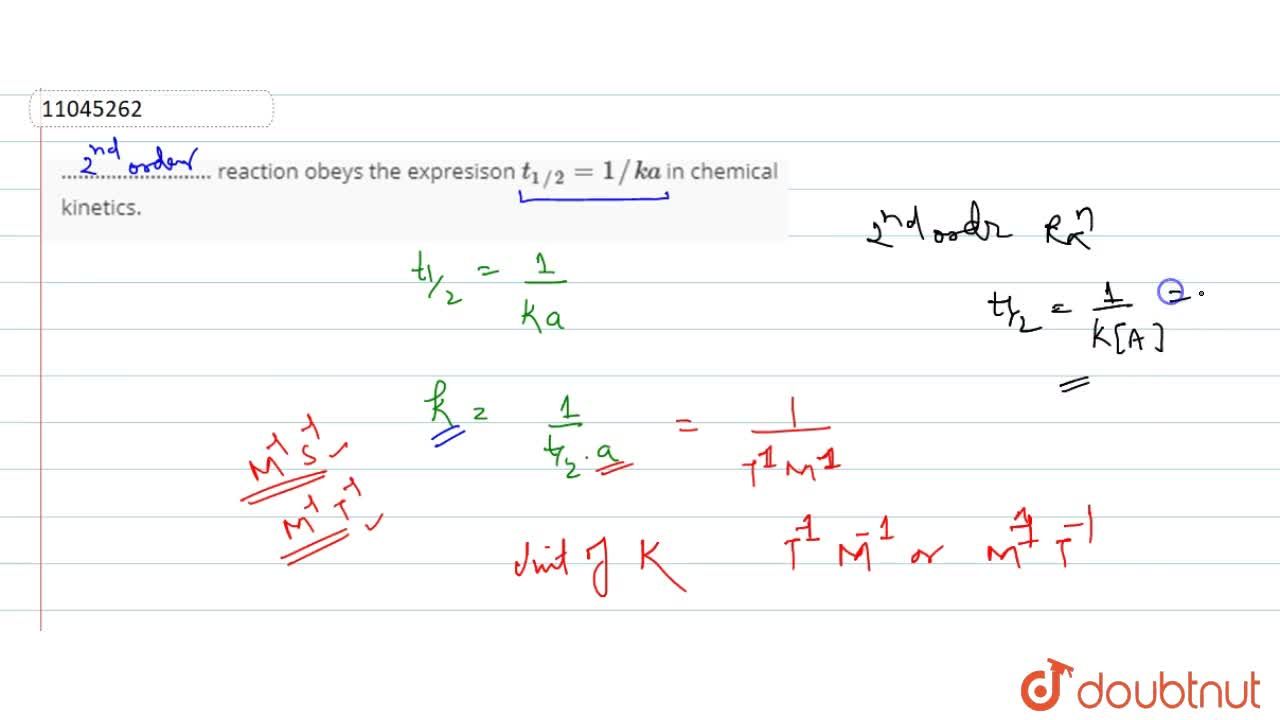
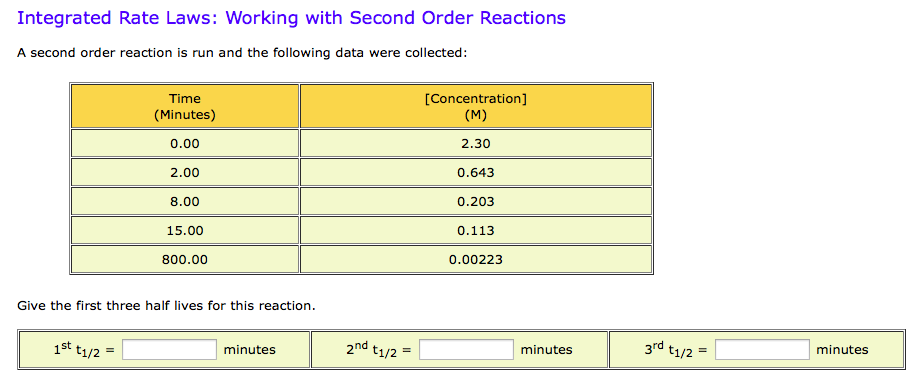
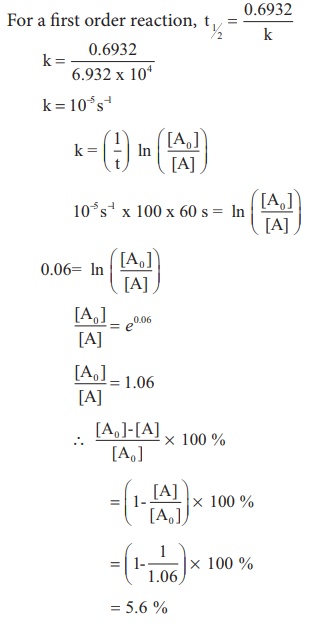
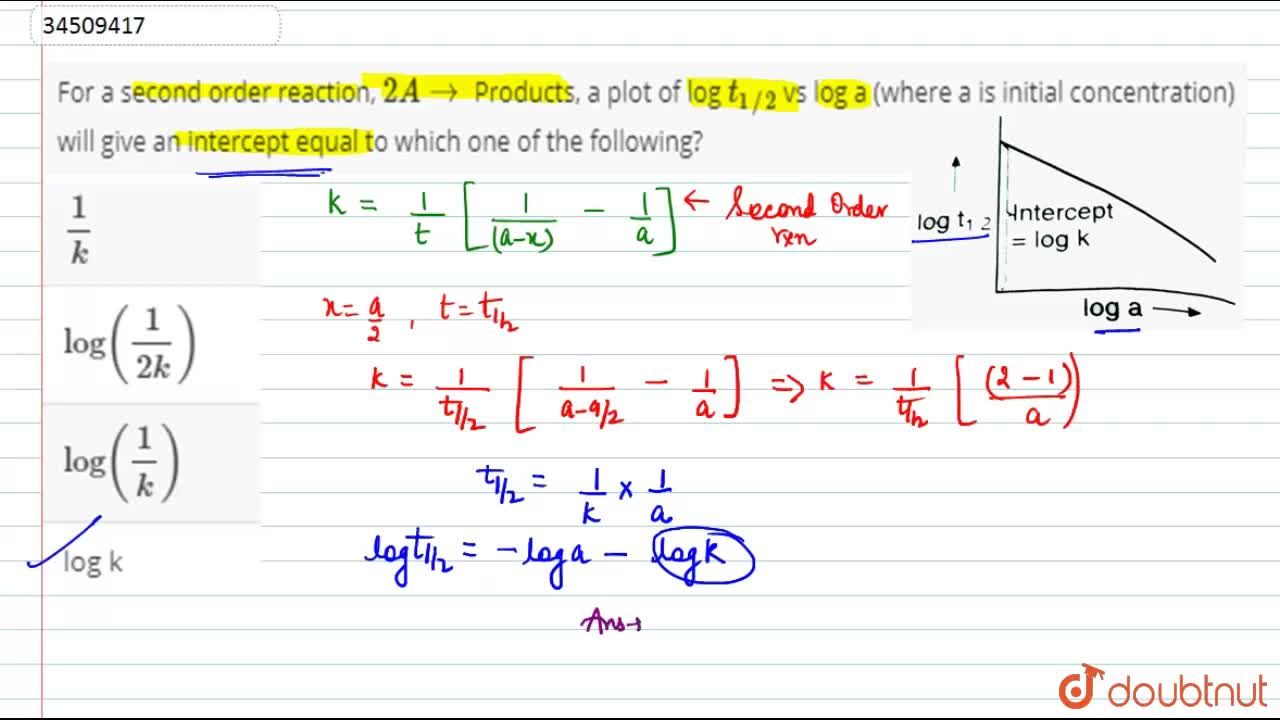
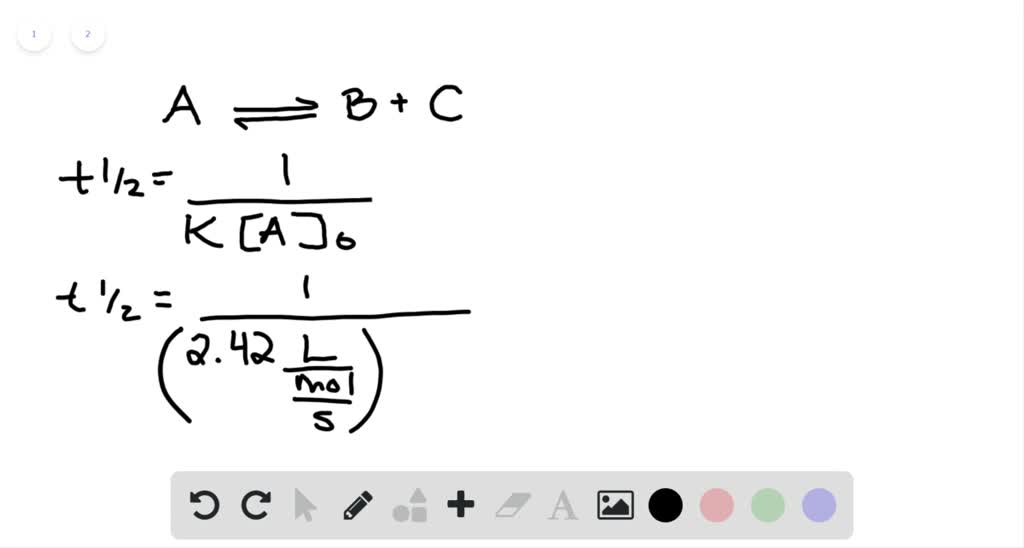
Expert Answer Option A cannot be correct answer Because it is the formula of half life of first order reaction Option B i View the full answer Transcribed image text For a secondorder
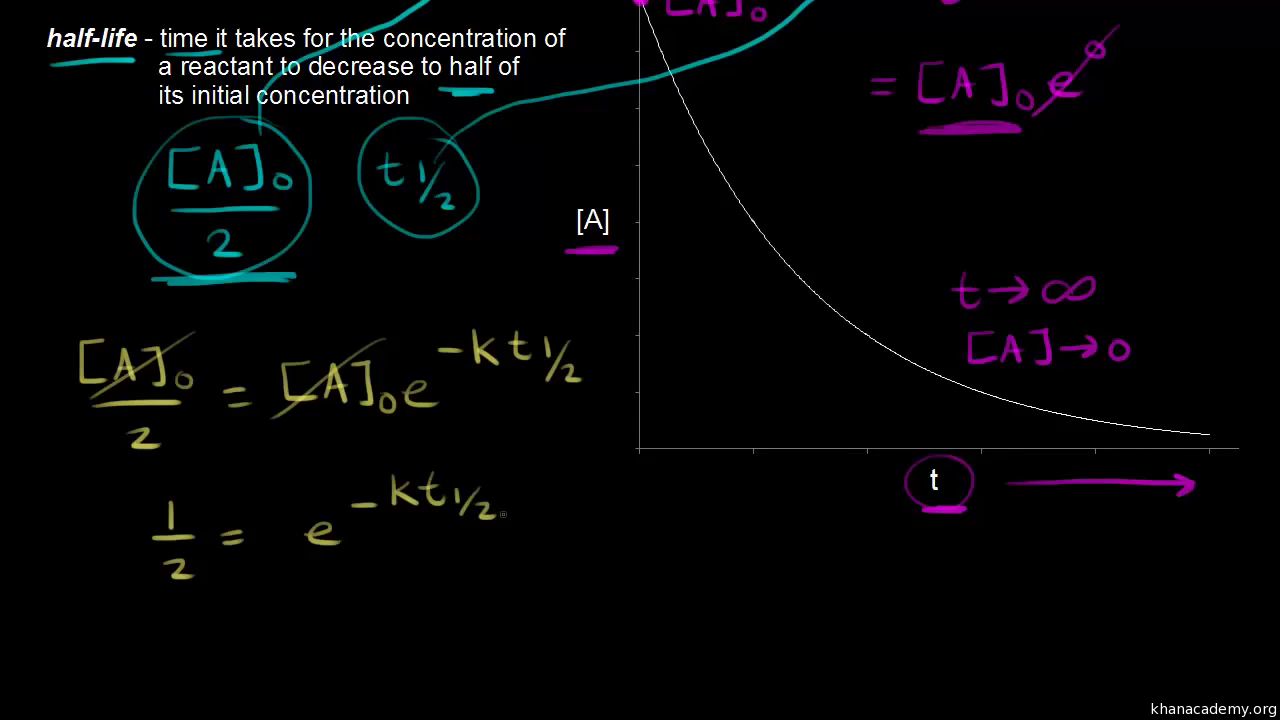
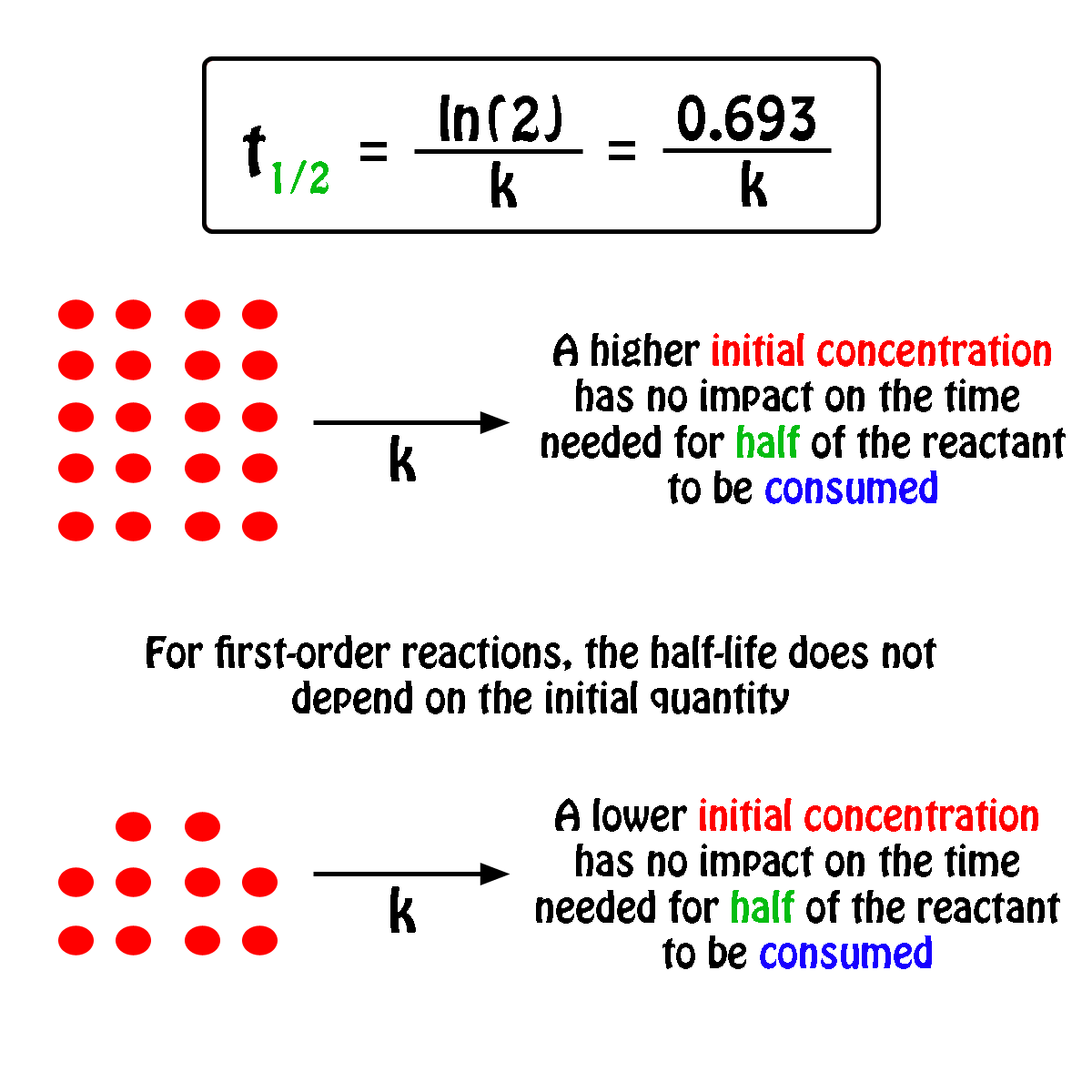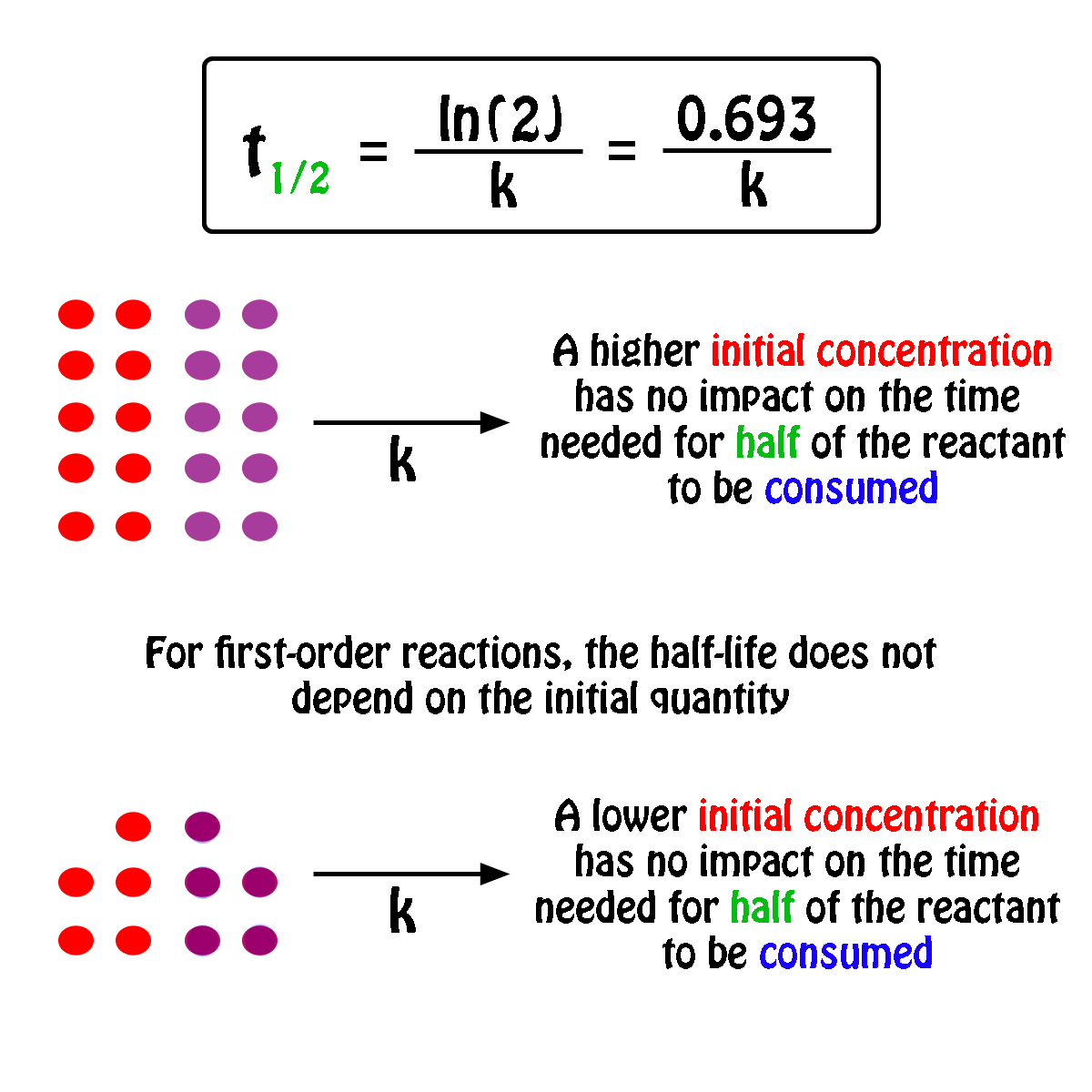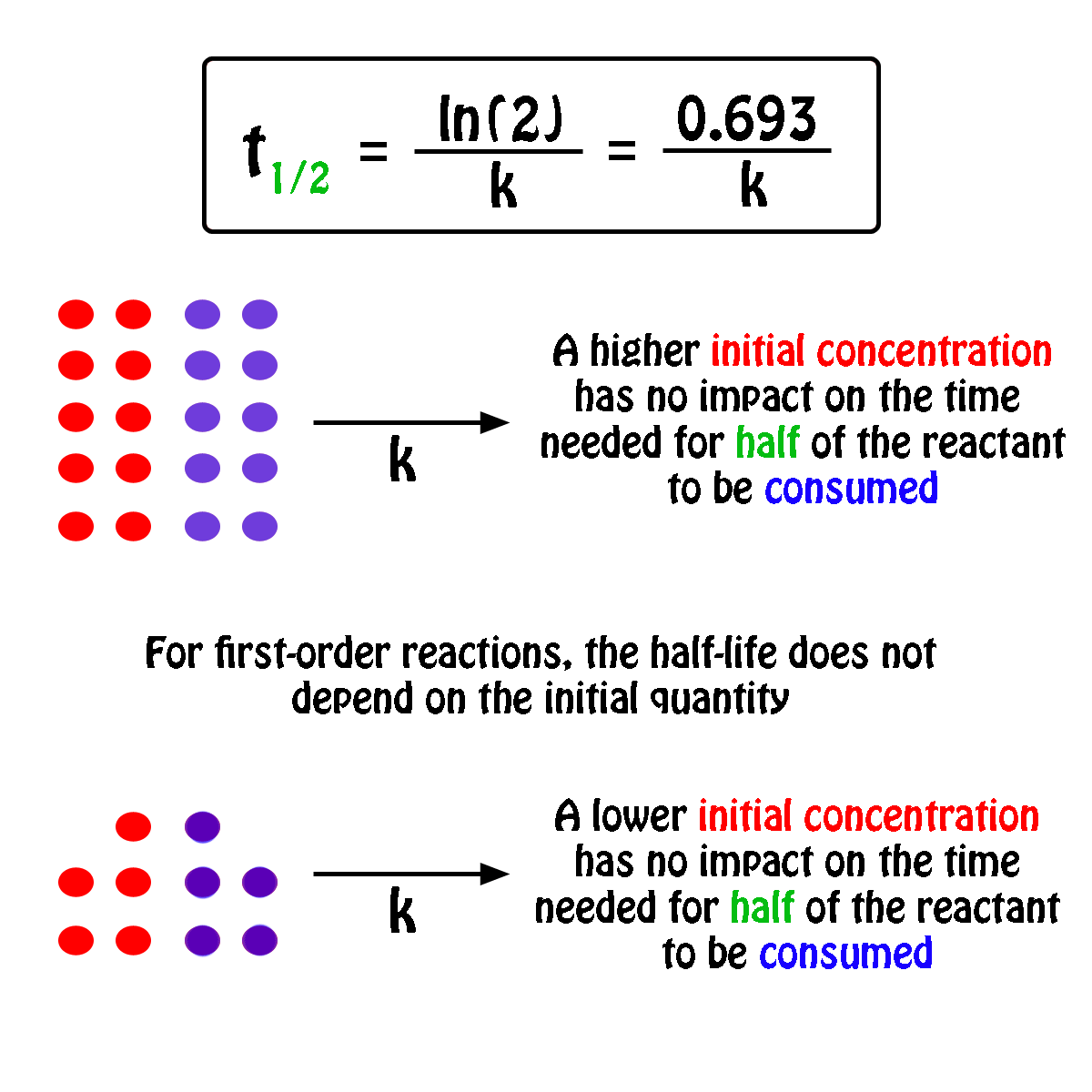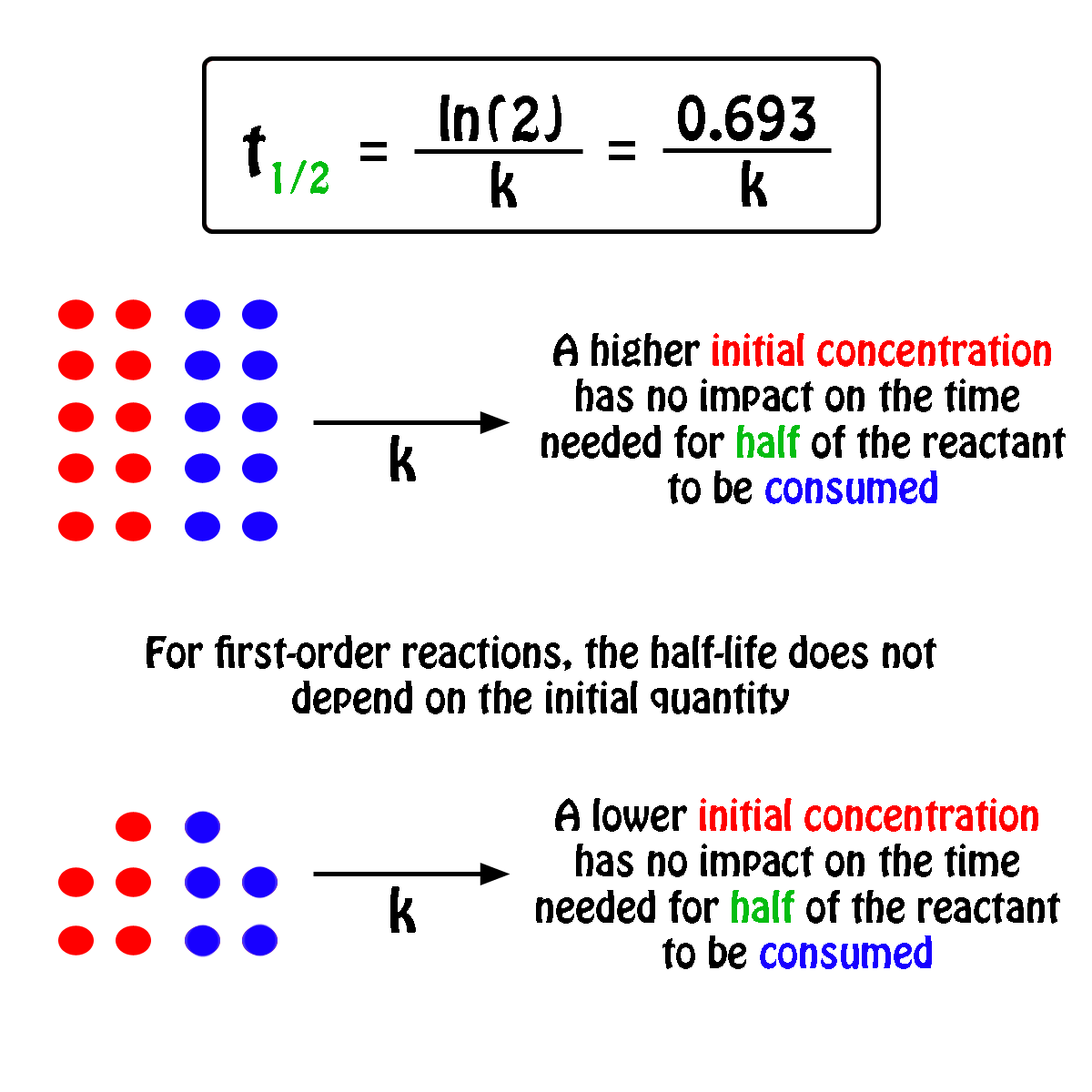
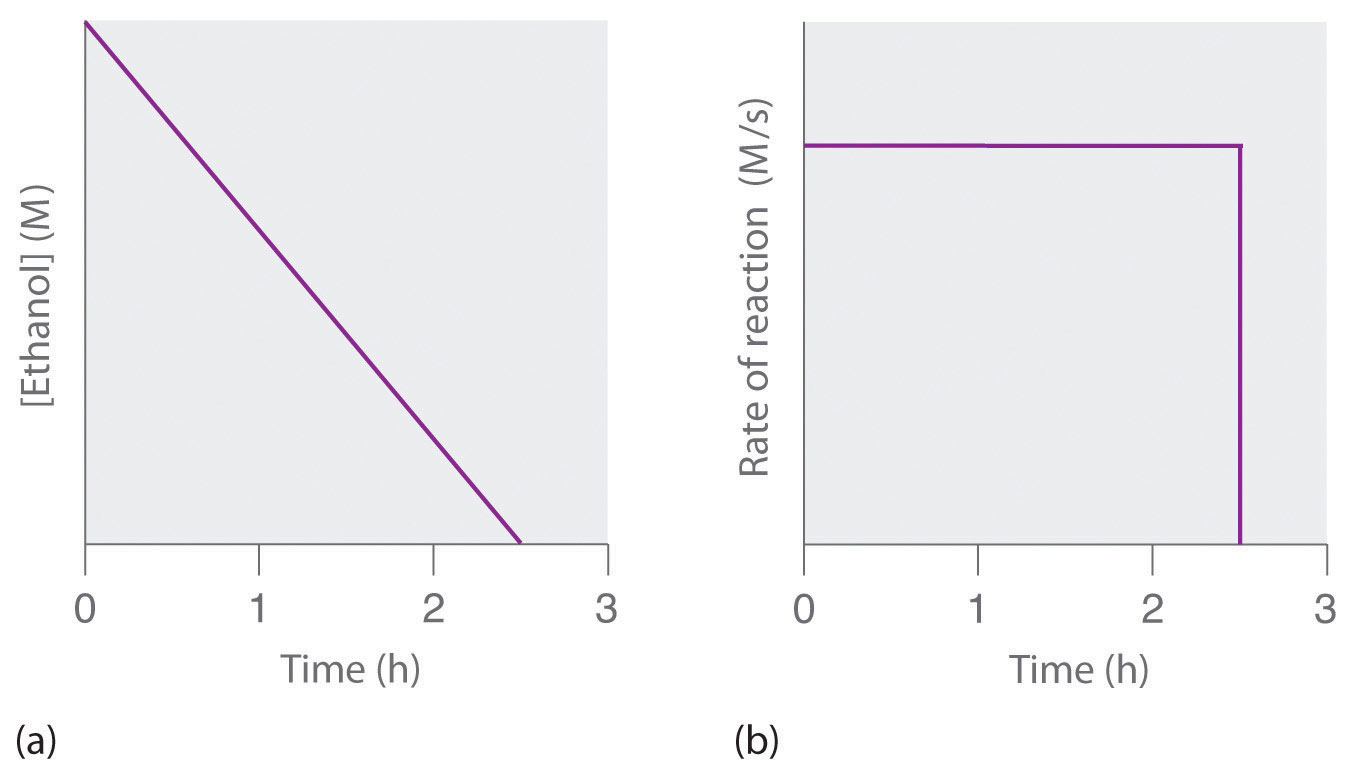
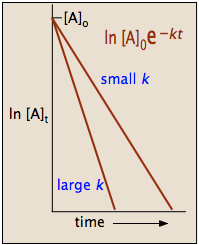
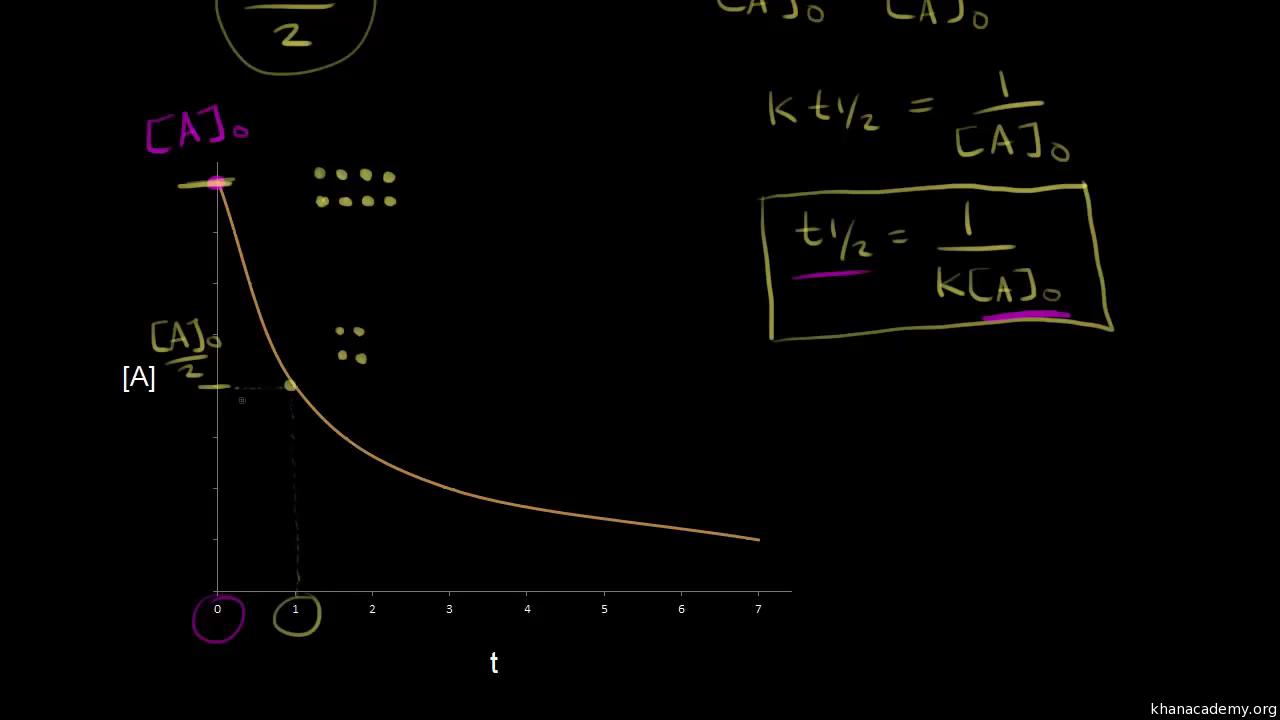
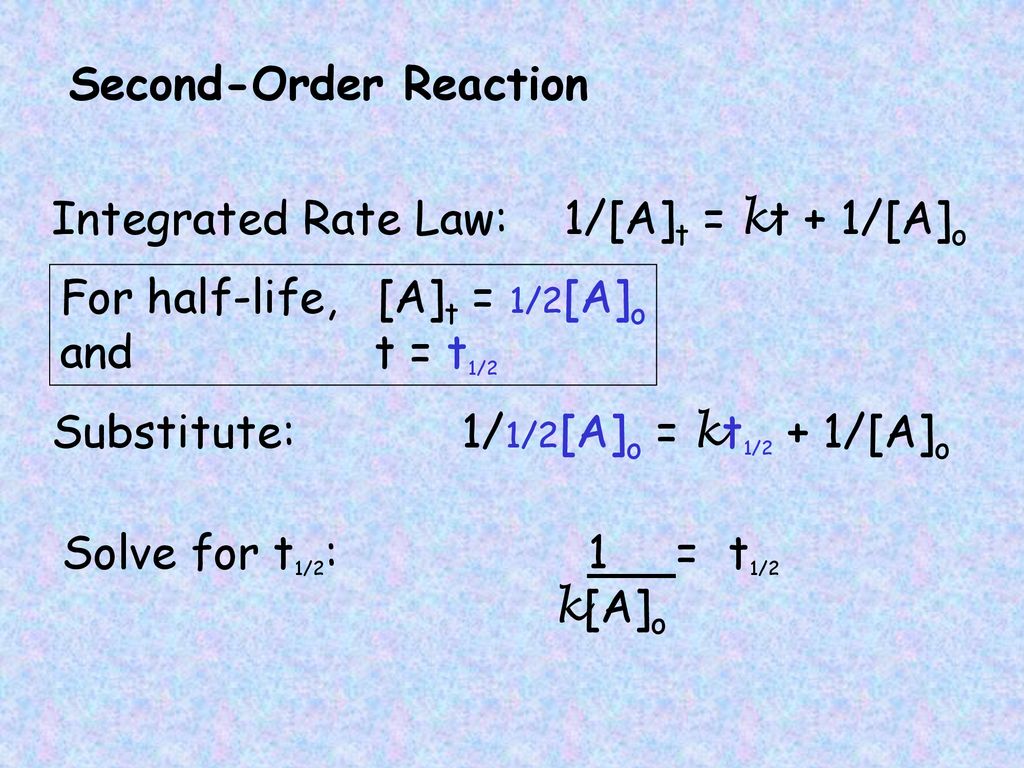
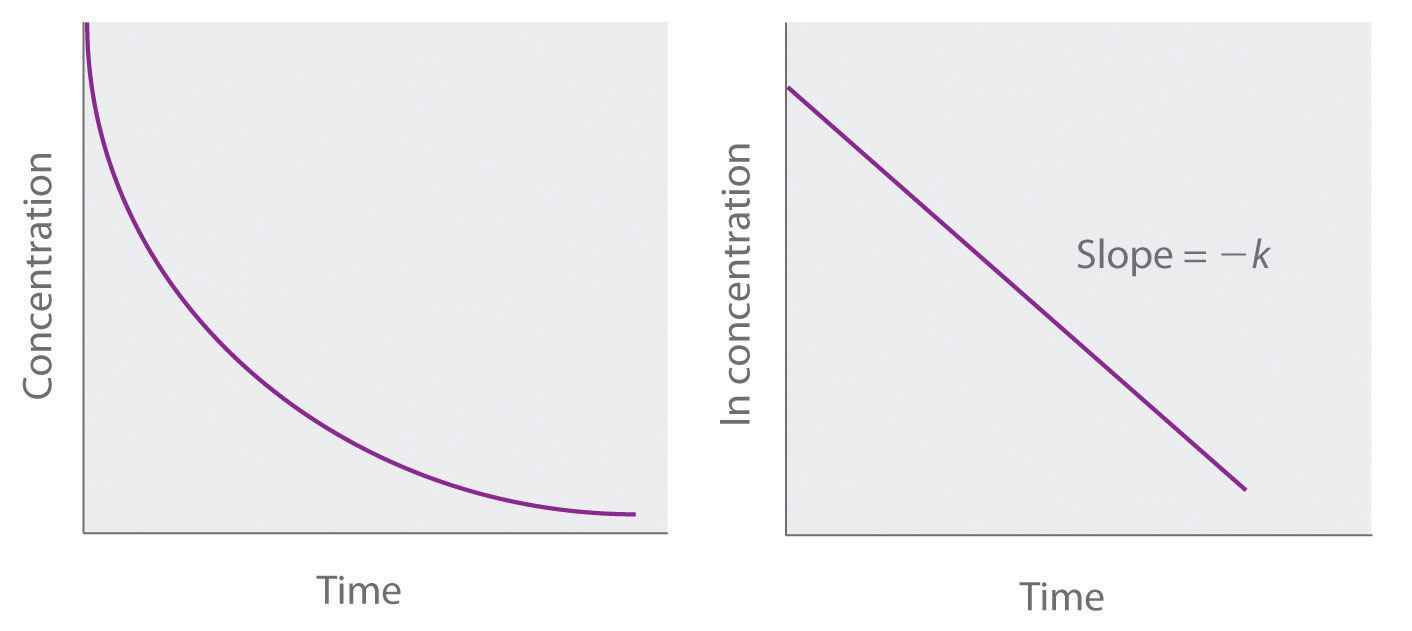
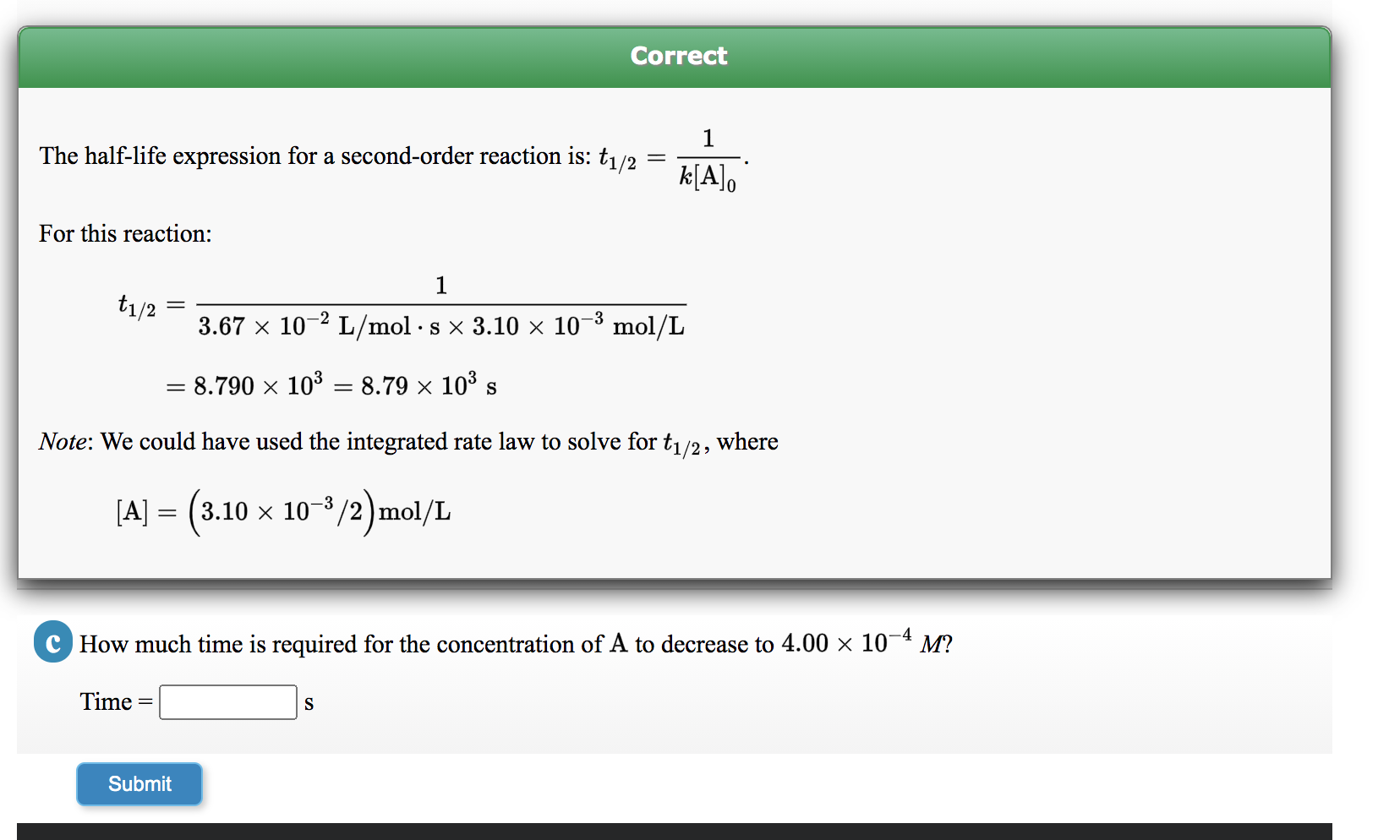
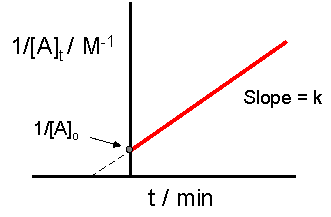
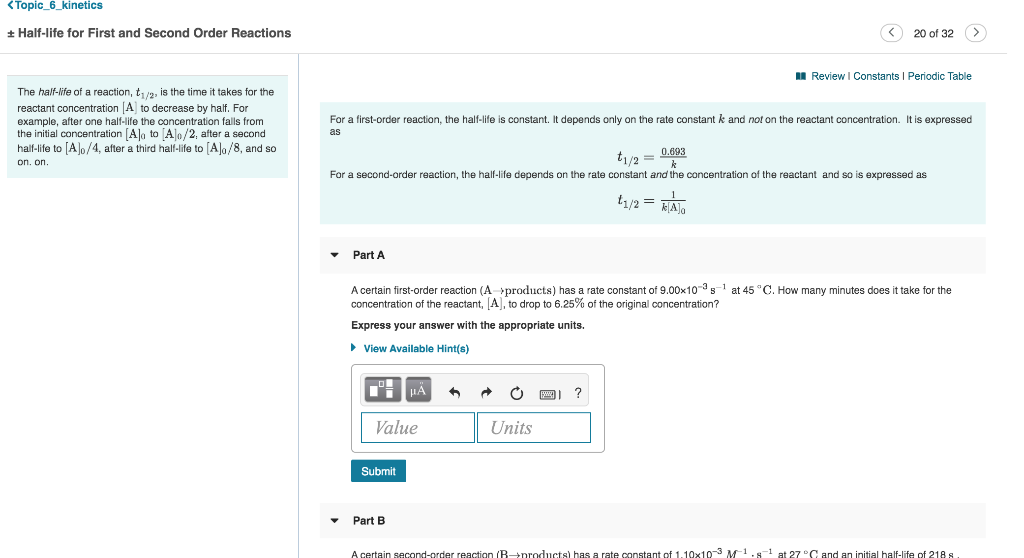
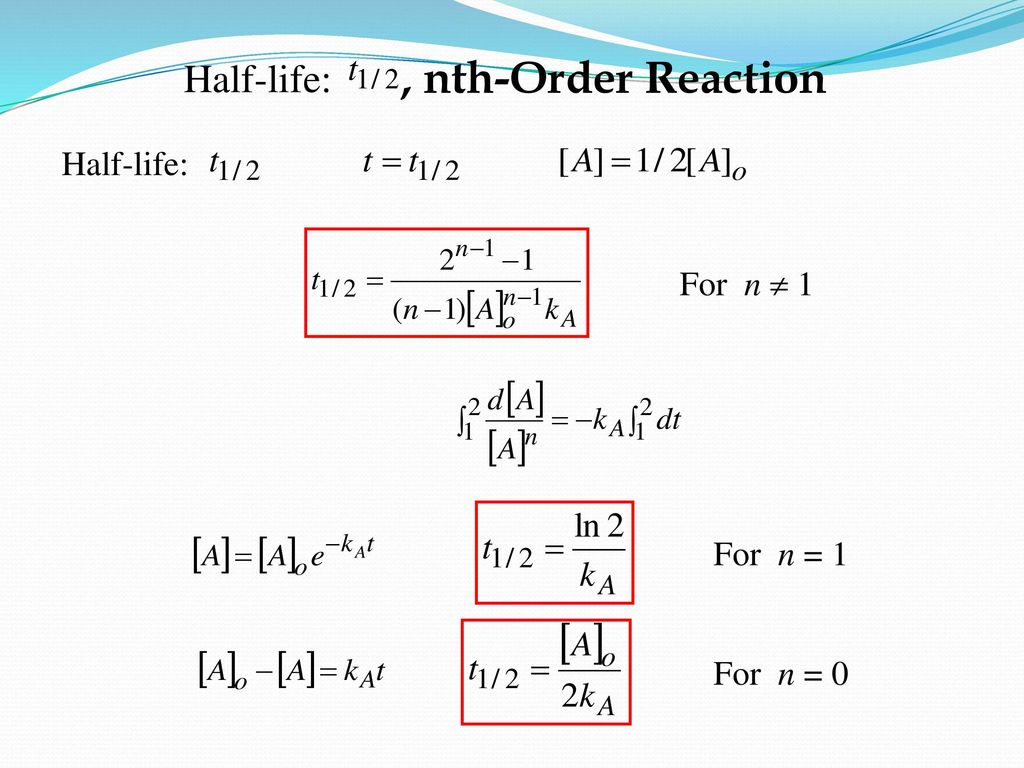
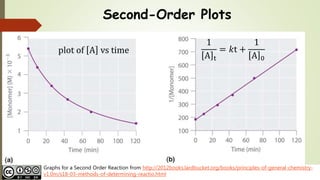
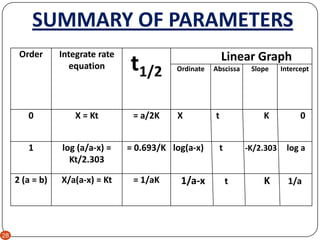
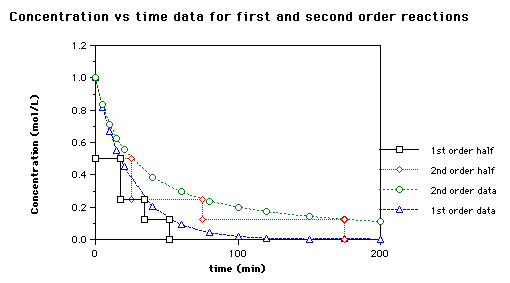
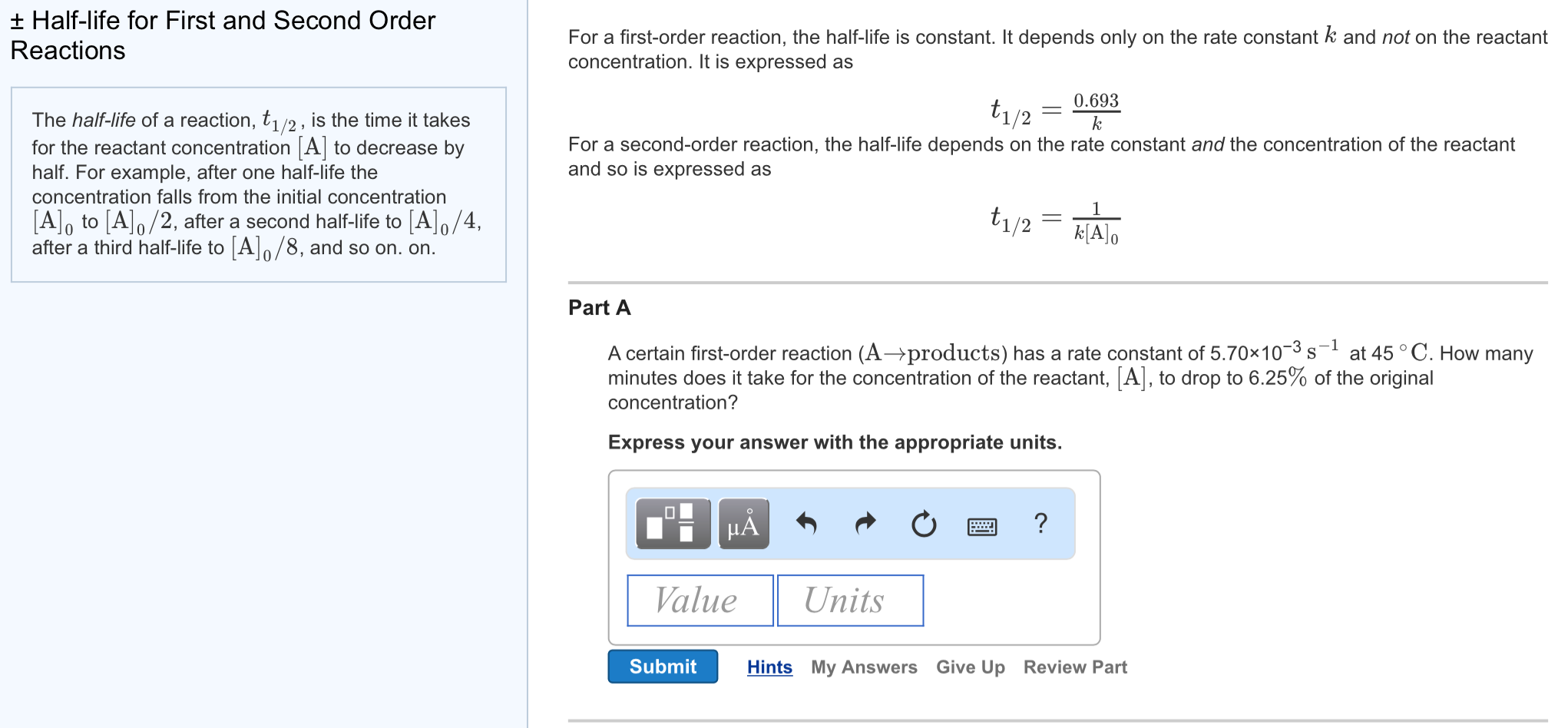
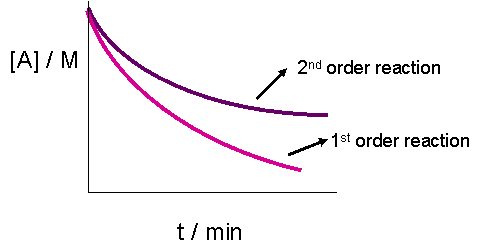
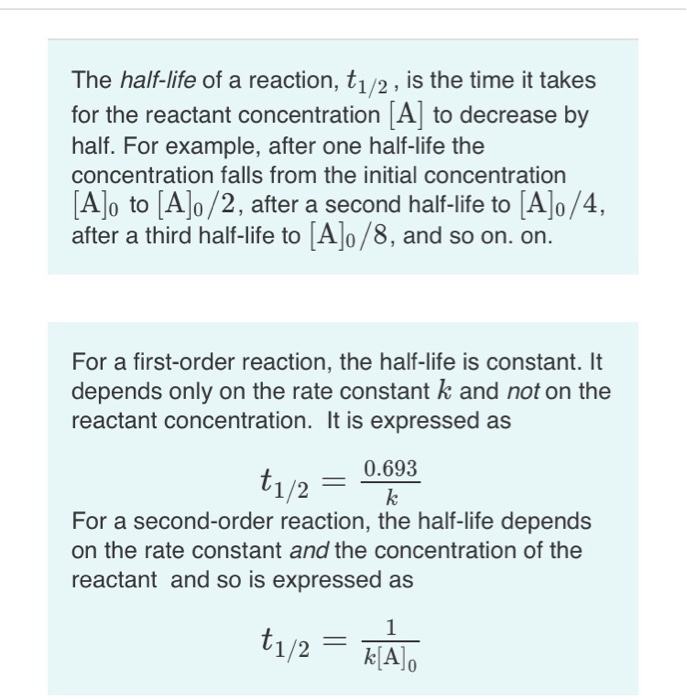
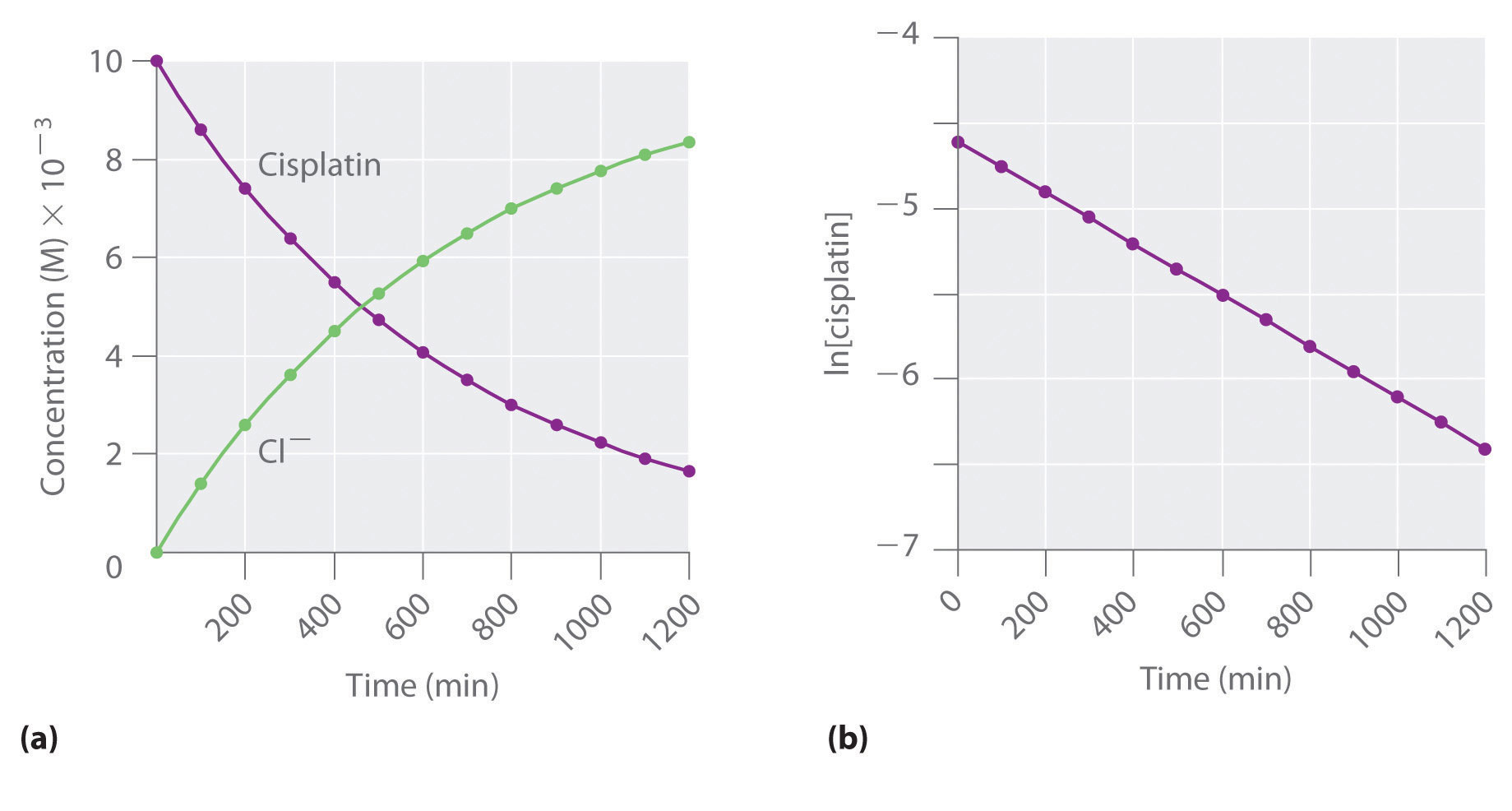
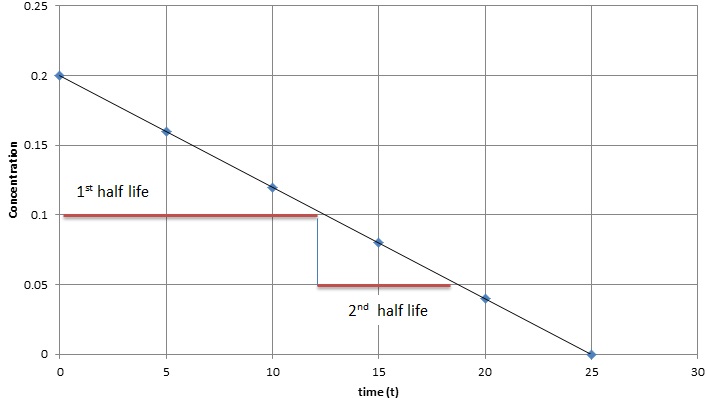
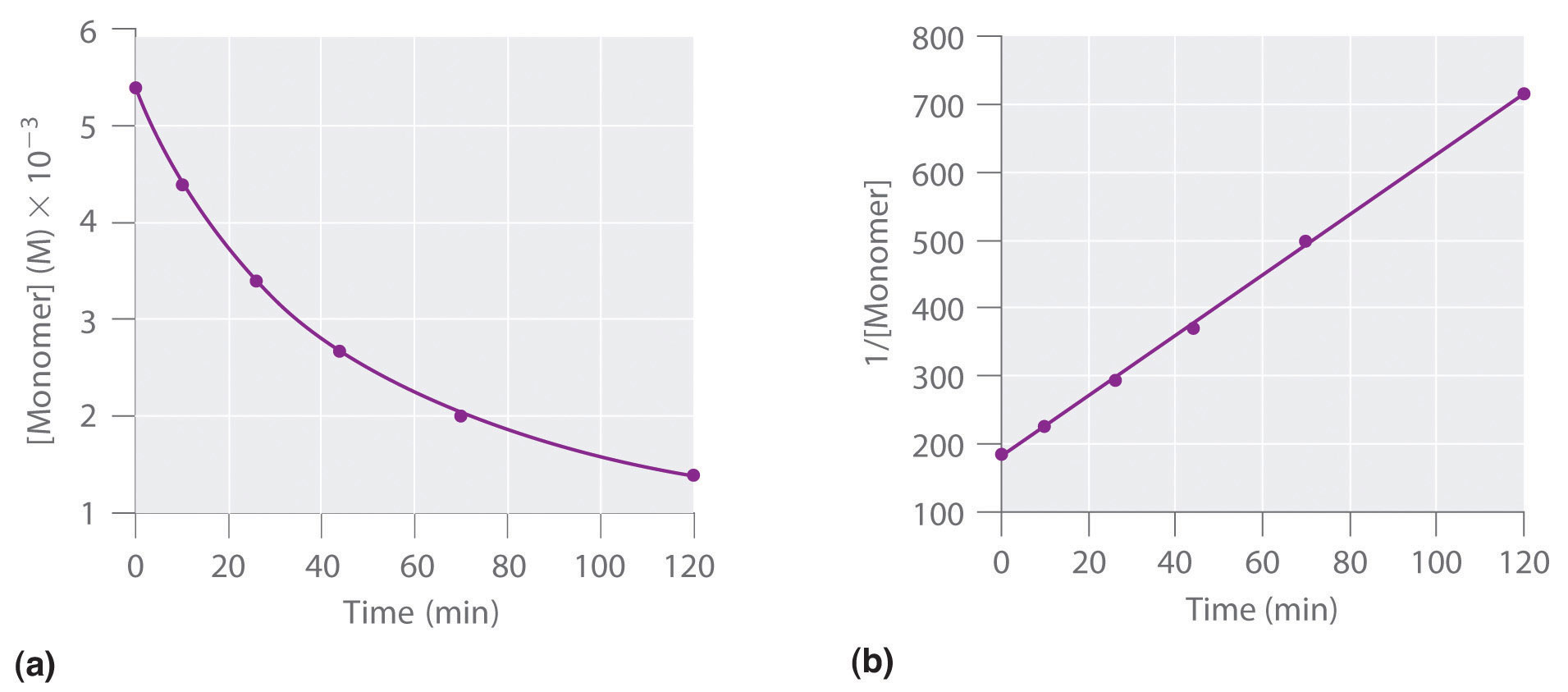
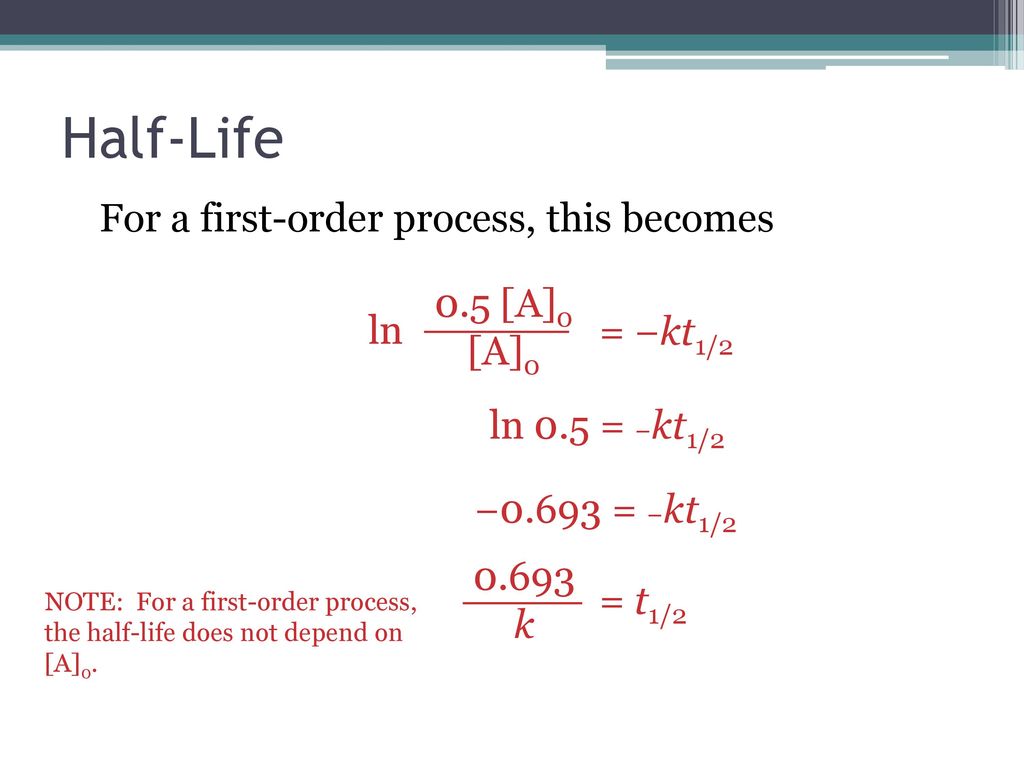
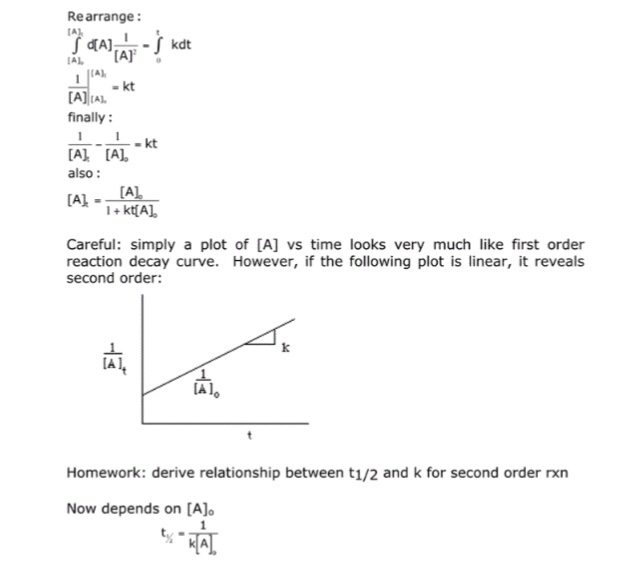
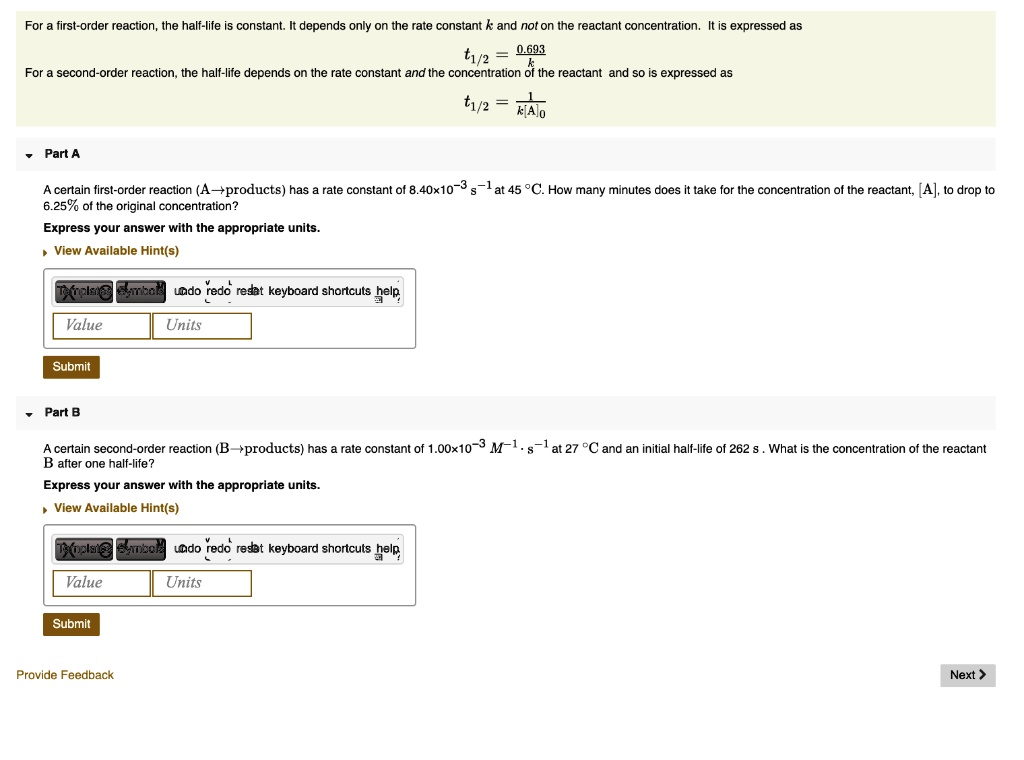
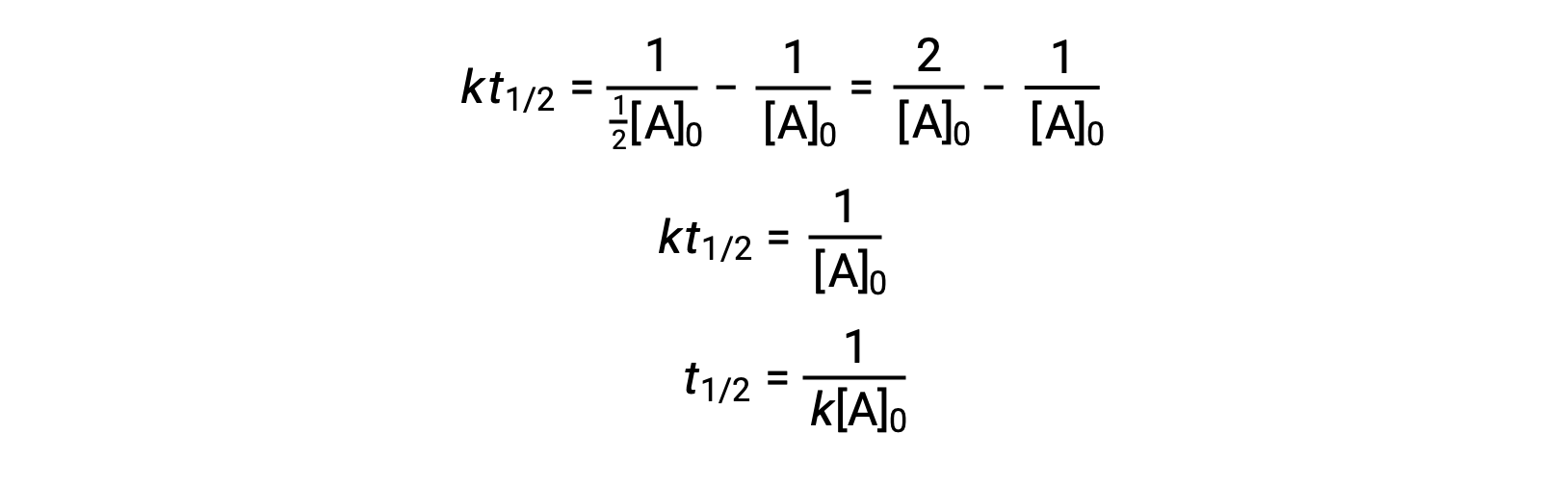
T1/2 for first order reaction-For n t h order reaction, the relationship between the half life period and the concentration is t 1 / 2 = k a n − 1 1 But t 1 / 2 = k a 1 Hence, n − 1 = 1 or n = 2T1/2=0693/k For a secondorder reaction, the halflife depends on the rate constant and the concentration of the reactant and so is expressed as t1/2= 1/kA 1 A certain firstorder
T1/2 for first order reactionのギャラリー
各画像をクリックすると、ダウンロードまたは拡大表示できます
 |  |  |
 |  |  |
 |  |  |
 | 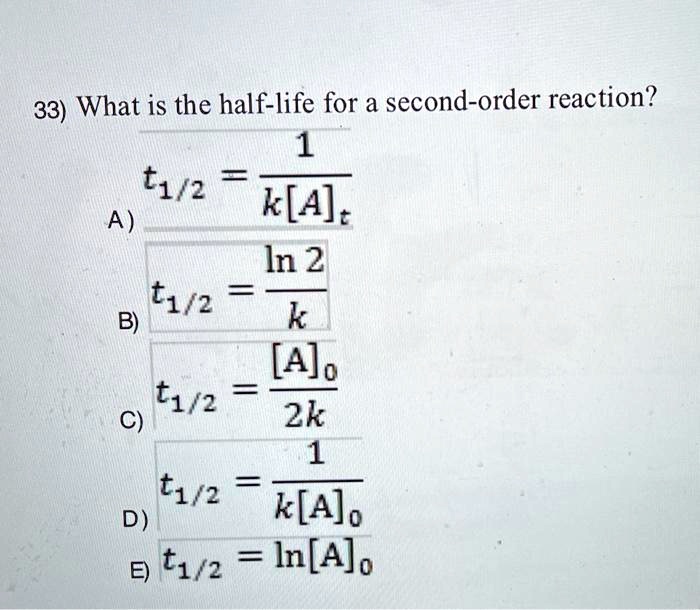 |  |
「T1/2 for first order reaction」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 |  | |
 |  | |
 |  |  |
「T1/2 for first order reaction」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 | 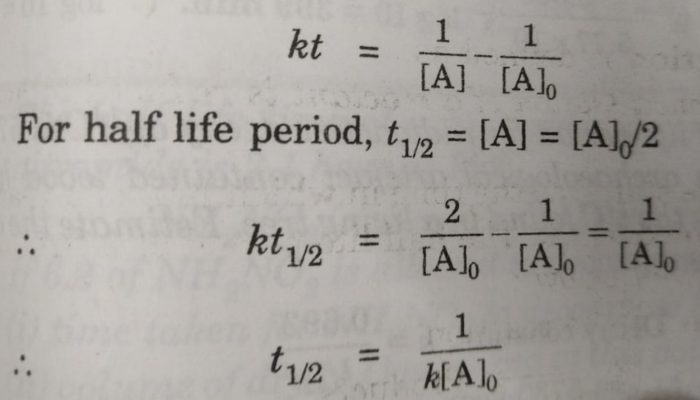 | |
 |  | |
 |  |  |
「T1/2 for first order reaction」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  |  |
 |  |  |
 | ||
「T1/2 for first order reaction」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 |  | |
 |  |  |
 |  |  |
「T1/2 for first order reaction」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 |  |  |
 |  | |
 |  | |
「T1/2 for first order reaction」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  | |
 |  |  |
 |  |  |
「T1/2 for first order reaction」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  |  |
 |  |  |
 |  | |
「T1/2 for first order reaction」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  |  |
 |
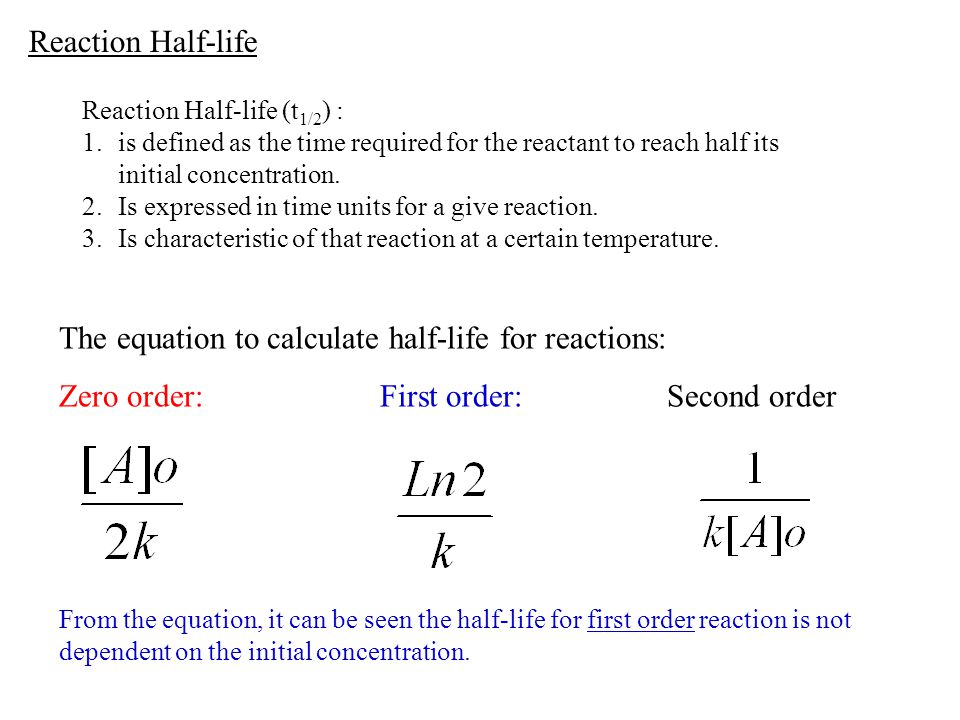
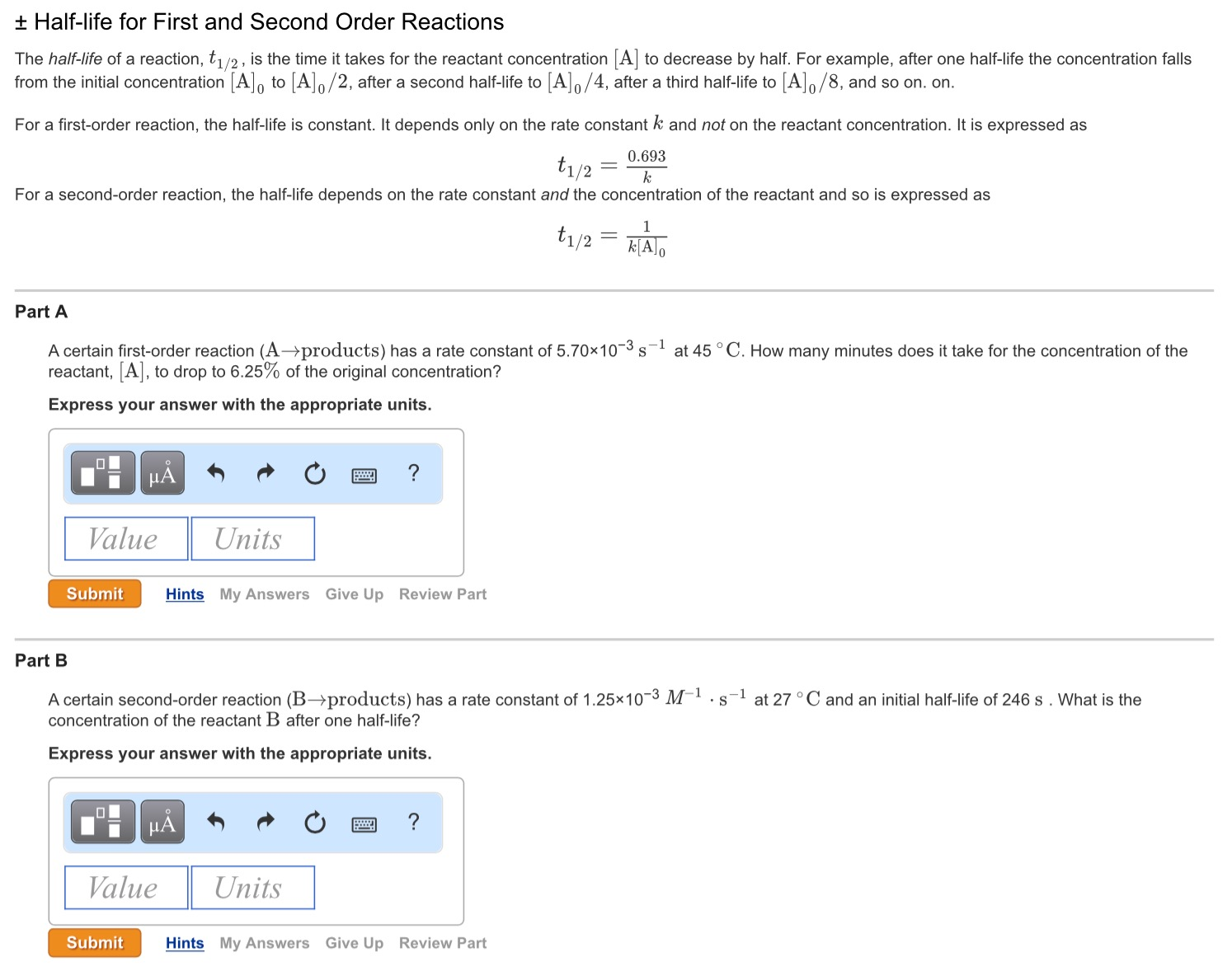
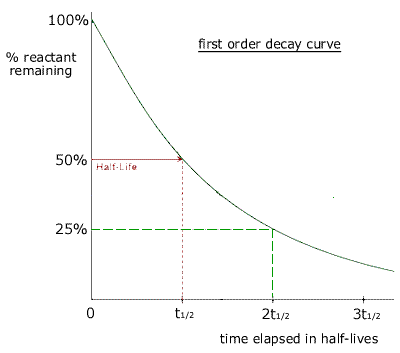
The halflife formula for various reactions is given below The mathematical expression that can be employed to determine the halflife for a zeroorder reaction is, t1/2 = R 0/2k For the firstMatch the effect of initial reactant on halflife with the appropriate reaction order t1/2 does not depend on reactantinitial t1/2 is inversely proportional to reactantinitial t1/2 is directly
Incoming Term: t1/2 for second order reaction, t1/2 for first order reaction, t1/2 for first order reaction is, t1/2 for first order reaction is 10 minutes, t1/2 for first order reaction formula, t1/2 for first order reaction is 14.26 minutes, derive t1/2 for first order reaction, the value of t1/2 for second order reaction is, value of t1/2 for first order reaction, t1/2 of a second order reaction decreases with increasing the initial concentration of reactant,



